As we continue to battle the coronavirus pandemic, we are once again reminded of the crucial role medical devices play in saving lives. Devices for emergency use such as ventilators, patient monitors, and infusion systems, but also seemingly ordinary pieces of equipment such as hospital beds, are used in many countries to treat particularly severe and critical cases.
A limited number of medical staff are treating large numbers of patients in critical condition with very limited medical-technical systems. Not only have devices been in short supply, but in many areas personal protective equipment has been scarce and has to be used beyond its intended lifespan. At the same time, medical professionals have been dealing with the added stress of not having sufficient knowledge about the disease and its symptoms.
In circumstances like these, the importance of intuitive and easy-to-use devices is greater than ever to decrease the strain on the already stressed personnel and to prevent errors that could damage patients’ well-being. By definition, usability not only facilitates the efficient and effective use of a product, but can increase user satisfaction and ensure the safe use of the device. Particularly in medical device technology, usability can contribute to reducing the number of deaths caused by medical errors.
Medical error as a cause of death
Medical error as a cause of death has been debated for many years. “To err is human”, a study published in 1999 by the Institute of Medicine (IOM), implied that up to 98,000 people were dying every year due to medical errors. More recent publications, for example, in the Journal of Patient Safety in 2013 or the Biomedical Journal in 2016, estimate medical errors to be the third most common cause of death ranging from 250,000 [1] to 440,000 [2] cases per year in the U.S. alone.
Some of the most common types of medical errors include: medication errors, errors during anesthesia, hospital-acquired infections, delayed, false or missed diagnosis, avoidable delay in treatment, inadequate monitoring after a procedure, failure to act on test results, and technical medical errors. Several of these can be traced back to a use error resulting in death – reason enough to consider and mitigate them to the greatest possible extent.
Use errors as a cause of death
Per definition, a use error is an action or lack of action while using a medical device that leads to a different result than that intended by the manufacturer or expected by the user (IEC 62366-1:2015). And, when users are under stress, they are more likely to experience use errors.
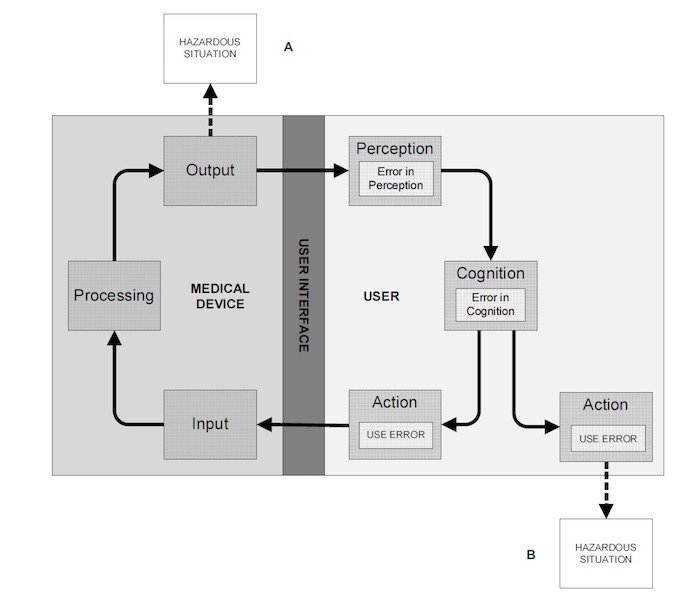
Figure 1: Model of user-device interaction (IEC 62366-1:2015)
A use error can indirectly (A) or directly (B) lead to hazardous situations that can harm the patient, the user or a third person, as shown in Figure 1. A use error might be caused by a user manual that is written in an incomprehensible manner or that includes figures that can be misinterpreted by the user and lead to the wrong action.
In the present context, a use error might involve a physician who has been working for 36 hours, not changing his mask correctly, i.e., not removing it aseptically. This use error leads to a hazardous situation: The physician has the potential to become infected with the coronavirus himself. The potential harm of contracting COVID-19 can affect the physician, his family and his future patients. We can only speculate that use errors have contributed to the death toll of the coronavirus pandemic; however, there is no data yet on this matter.
Shortcomings in the usability of medical devices also contribute to medical errors (see Figure 2).
Figure 2: Examples of shortcomings in the design of a user interface
The design of a medical device requires usability engineering to ensure its safe use. To support this, international authorities have published standards and guidance documents, providing information on how to establish, document, implement, and maintain a usability engineering process.
Usability engineering as a key to patient safety
The usability engineering standard IEC 62366-1:2015 describes how a manufacturer can analyze, specify, and evaluate the usability of a medical device, and develop a product as it relates to safety. The principle of the process is to involve the potential user groups early on and follow an iterative approach. As part of the overall risk management, the usability engineering process focuses on how use-related hazards and risks can be assessed and mitigated while using a medical product.
As a supplement, the IEC 62366-2 standard provides additional guidance on how the usability engineering process can be established and implemented in the overall design and development process of a medical product. Beyond safety, the guideline specifies how usability engineering can be used to increase efficiency and user satisfaction in order to generate additional competitive advantages.
Figure 3: Information for use as part of the user interface
The overall goal of the process is to create a medical device with a user interface that is easy, intuitive, and safe to use. By definition, the user interface of a medical device is the means by which the user and the medical device interact. This includes hardware and software elements as well as visual, tactile or auditory feedback. The information for use, also called accompanying documentation, is also part of the user interface of a medical device and encompasses additional materials such as information for safety, training materials and quick reference guides.
The information for use must be written in a user-friendly manner and the manufacturer must ensure that this information is perceivable and understandable for the intended user, supporting the correct use of the medical device in the intended use environment.
In addition to creating a device that is safe, efficient and easy to use, usability engineering offers the manufacturer the following advantages:
- Reduced time to market
- Lower development costs
- Improved sales
- Intuitive use and reduced training efforts
- Less demand for customer support
- Reduced effort for writing information for use
- Less susceptibility to product liability claims
The scope of the usability engineering process and the effort required depend on the product and its complexity and classification as well as on the timing of market launch.
The usability engineering process is an iterative process that should be integrated into the overall product design and development process. The objective of usability engineering is to gain a good understanding of users’ needs and to include them in the product specifications to ensure that the product meets the requirements at the time of its market launch. In the field of technical writing, this step is comparable with the information management process described in chapter 6 of IEC 82079-1:2019.
Understanding user needs
Figure 4: Contextual inquiry as a method to analyze user needs
Understanding user needs is the basis of the usability engineering process and should be a part of product development from the start. At the beginning of the ideation phase, when a new product is being conceived, manufacturers should carry out a contextual inquiry to understand what users are doing now, i.e., without the product to be developed or with its predecessors. This means looking at products and processes currently in place: who is doing what, which patients are being treated, where are patients treated, how is the current product/process interacting with the patient’s body?
Together with the general operating principle and the definition of the intended medical indication of the device, this information forms the use specification as a basis for the development of the medical product. Similar aspects are specified in IEC 82079-1, chapter 6.2.1 – 6.2.2, for the analysis and planning of information. The analysis should result in an understanding of the context of use and the nature of the target audience. For the design of information for use for, let’s say, a new implantation kit, the results could include that the product is always used in a sterile environment and that various user groups with varying qualifications perform different tasks with it.
Designing the user interface
For further development and to start the conceptual design of the product, the manufacturer needs to start with the user interface specification. We need to translate important requirements from the use specification and the contextual inquiry into measurable specifications. The focus is on the hardware and software interface, but it is also at this stage that we need to specify the availability of information for use and any kind of training.
Referring to the use specification example from above – a sterile environment – an electronic manual provided on a tablet that can be easily covered and cleaned after use can be a resulting specification. Furthermore, several versions of the information for use might be available, e.g., a quick reference card with several images to show the cleaning staff how to clean and disinfect the implantation tools, information for use for the nurse on how to safely unpack an implant, and implantation information for the surgeon.
To ensure the safe use of the device, the manufacturer needs to analyze the potential risks that can occur when using the device, also including the contributing factors defined in the use specification. This analysis must take into account hazards and risks that may affect patients, the user or any third parties. For the purpose of usability engineering, this part of the risk management should focus on the use-related hazards and risks, e.g., caused by use error. Additional specifications of the user interface could be the result of the use-related risk analysis, ensuring proper mitigation of potential risks. Adequate risk mitigators reduce the risk first and foremost through inherent design. It is also common to see information for safety in the accompanying documents cited as a mitigation, but this is not a recommended method.
To get back to our example from above, an implant should be packed safely in primary and secondary packaging, ensuring sterility. The labeling of the packaging needs to ensure that the nurse receives all the important information, e.g., the length and diameter of the implant, to select the right product for the individual patient. Also, the quick reference guide must include proper information on which disinfectant to use and how to assemble and disassemble the implantation tools.
Defining use scenarios
Once potential risks and hazards have been determined, the manufacturer begins describing typical use scenarios. A use scenario is a sequence of tasks performed by a specific user in a specific use environment. Those leading to harm or a hazardous situation are called hazard-related use scenarios. Use scenarios are important for understanding the typical workflow and aspects that can influence device use, e.g., the professional skills as well as the mental, physical or demographic characteristics of the user, the conditions within the use environment and any possible distractions.
A detailed user interface specification, including the input from the risk analysis and the defined use scenarios, provides a solid basis for the product designer to create a product that is easy and safe to use and that meets the needs of the user. Comparable requirements for the information for use are specified in IEC 82079-1, chapter 6.2.3 – 6.2.7. These help to decide, for instance, what kind of media to use, if the information needs to be segmented for various audiences or languages, and how the information will be provided (online or printed, one document vs. a series of documents, with the product, on the product or separately). It is helpful to collect further feedback with regard to the information sources and information maintenance. Chapter 6.2.7 of IEC 82079-1 also requires a risk management process with similar principles and a focus on the information for use.
Back to our implantation kit, the use scenarios can focus on the selection of the kit, the unpacking and provision of the sterile implant and the implantation tools, the implantation, and the cleaning and disinfecting of the reusable implantation tools.
The design process
Figure 5: Conceptual design providing several ideas for a new product based on user needs
Quite often the outcome of the conceptual design is not a single concept, but a variety of competing ideas of how the new product could look. As not all of the ideas can be realized, we need to make a decision as to which idea will be pursued further. Ideally, the decision is not made by the team of developers and product managers alone but involves different user groups. A good methodology is to conduct focus group discussions, expert panels, or individual interviews with a selection of users from different user groups.
The outcome that is provided to the user is often a hybrid of concepts rather than a single design concept. After combining the strengths and eliminating the weaknesses of the various concepts that have been presented to and discussed with users, we can create a solid basis for further design activities. The development team can be sure that the design meets the expectations of the user.
In an iterative design process, the concept will be further detailed and improved. In the meantime, we can carry out additional usability evaluations to ensure the design is evolving in the right direction. The information for use and any training material should also be part of the iterative design and development process, including evaluations by users. Because these usability evaluations contribute to the design input, they are called “formative usability evaluations”.
It may be beneficial to plan a series of usability evaluations at the beginning of the development process, describing the objective of the evaluation and the method to be used to obtain feedback on the design. Involving representative users may also be helpful.
Summative usability evaluation
At a certain point, further improvements will be neither necessary nor reasonable. This is the time for a design freeze, and the beginning of the realization and implementation phase. From a usability perspective, the summative usability evaluation is the last step before the usability documentation is completed. The summative evaluation is perhaps the most important activity, as it will provide evidence as to whether the product can be used safely and without unacceptable risks, and whether risk mitigators have been established successfully.
The summative usability evaluation must include typical user groups and be carried out in the typical use environment, which could be, for example, a hospital setting or a home. The summative evaluation plan defines the details of the usability evaluation. Hazard-related use scenarios must also be part of the planning to describe the actions to be performed by the user in the simulated setting. Typically, the use scenarios are translated into test scenarios. To demonstrate that all risk mitigators have been evaluated in terms of their effectiveness, it is important to ensure traceability between the use-related hazards and risks, and the test scenarios in the summative evaluation plan. During summative evaluation, we need to address all parts of the user interface such as user manuals, quick reference guides, training and materials for training, or any other accompanying documentation.
Similar guidelines can be found in chapter 6.3 of IEC 82079-1: “Design and development, including review, editing, and testing”. Here, usability testing is also mentioned as an empirical method for comparing competing versions of the information for use, and to evaluate their comprehensibility and usability.
Once the summative evaluation has been accomplished successfully, the usability engineering process can be completed by documenting all activities in the usability engineering file. This documentation will be part of the overall technical documentation of the product that is submitted to the authorities.
Increasing patient safety and user satisfaction
Including usability early on and involving representative users throughout the development process not only helps to ensure a product that satisfies the user’s needs and expectations, but also a product that they can use easily and safely.
Figure 7: Summative usability tests with the final product in the typical context of use
In this way, usability engineering can be the key to increasing user satisfaction and patient safety, even in times of high stress levels and in unfamiliar circumstances such as we are currently experiencing in the coronavirus pandemic. We need to ensure that medical practitioners can give their full attention to the patient and not have to focus on how to handle a medical support device.
References
[1] Based on a John Hopkins study and published in the Biomedical Journal in May 2016
[2] Patient Safety America, published in the Journal of Patient Safety in September 2013